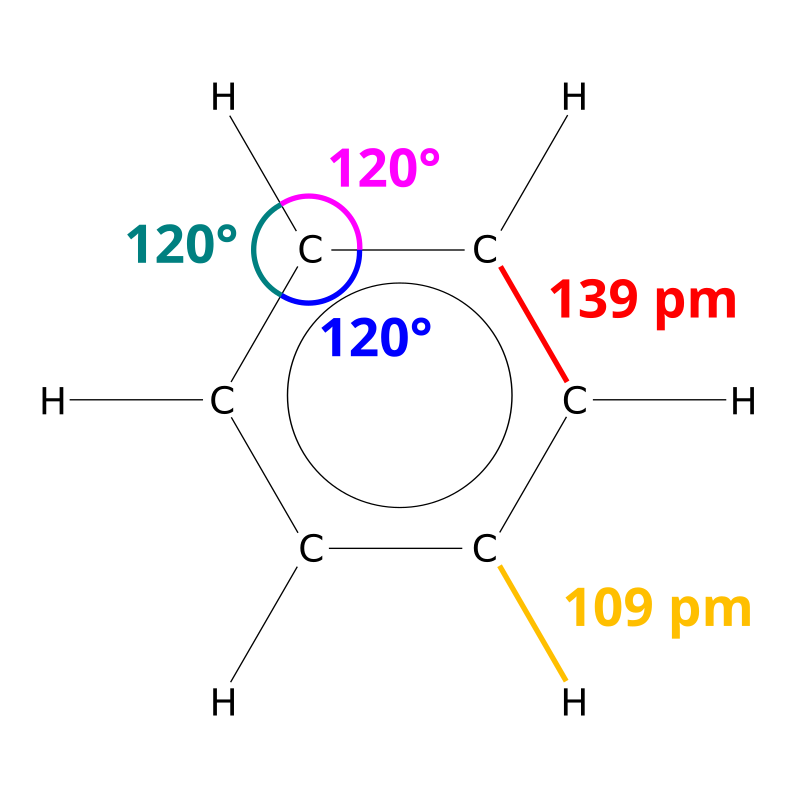
Benzene is an aromatic hydrocarbon containing alternative double and single bonds. Benzene is more stable due to resonance stabilization. It follows Huckel’s rule. it is a cyclic conjugated species i.e it contains alternative double and single bonds. The main source of benzene is coal tar. This was first isolated by Farady from the cylinders of compressed illuminating gas. Hoffmann obtained benzene by fractional distillation of coal tar. in the benzene all the carbon atoms are sp2 hybridized. The number of sigma and pi bonds is 12 and 3. The C-C bond length is 1.39A and C-H bond length is 1.09A.
Benzene can be prepared from
From Acetylene
From sodium benzoate
From phenol
From Chlorobenzene
From Benzene sulphonic acid
From benzene diazonium chloride
From Grignard Reagent
Chemical properties
Addition reaction
Electrophilic substitution reaction
Oxidation reaction
The detailed reaction and mechanism will be provided in the next blog of benzene.
Thank you Very much.

I am your student
Can you sand me the link of all named reaction
Yes, please send me your email
By mail